MAGNETIC RESONANCE IMAGING
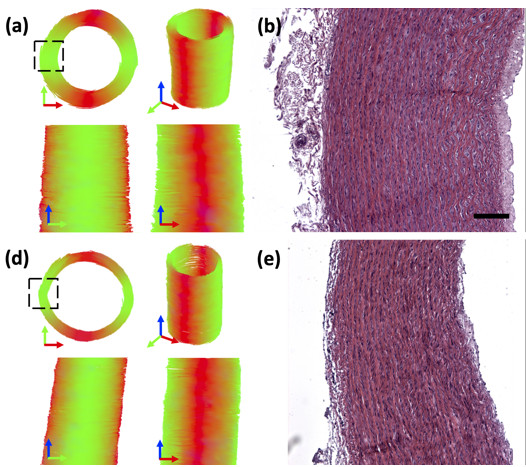
Diffusion tensor imaging in arterial tissue:
Work in our lab has focused on investigating diffusion tensor imaging within arterial tissue. Initial studies highlighted the sensitivity to cell content in porcine carotid arteries – specifically that the despite maintaining native arterial tissue microstructural arrangement in decellularised arteries, the ability to measure anisotropic diffusion is lost without the presence of smooth muscle cells1.
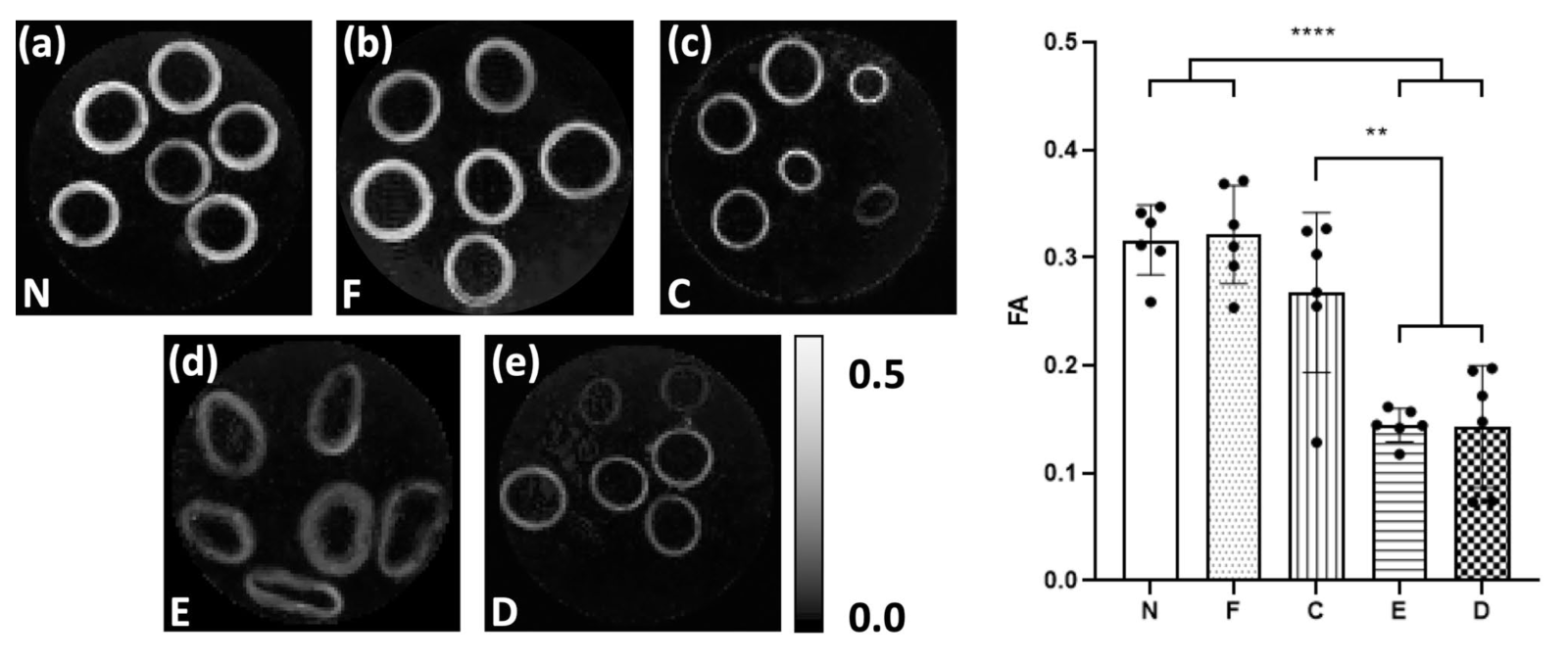
Figure 1. Parametric maps of FA in a representative slice for each of the tissue models. As measured in vessel media, both (a) native (N) and (b) fixed native (F) PCaA showed significantly higher FA than both the (d) elastin degraded (E) and (e) decellularised (D) tissue models. (c) Collagen degraded PCaA also showed a significantly higher FA than both elastin degraded and decellularised PCaA. FA maps scaled to show 0 to 0.5 (**p =0.0018 (C vs. E), **p = 0.0016 (C vs. D), ****p<0.0001.
Moving towards more clinically relevant human tissue, we also investigated excised human carotid arteries with varying degrees of atherosclerosis. DTI-derived metrics showed a sensitivity to early-stage atherosclerosis (the thickened intima) and late-stage (the lipid core). By combining image registration and an unsupervised segmentation algorithm, we also identified the strong correlation between elastin content and FA and MD in atherosclerotic tissue2.
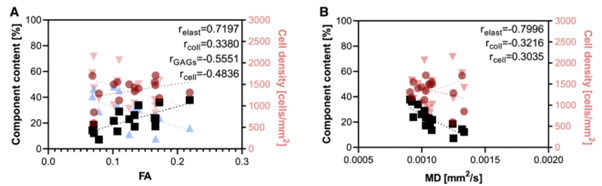
Figure 2. Correlations between microstructural components in excised human carotid arteries and DTI-derived metrics, (A) FA and (B) MD. Elastin (black squares) has a strong correlation with FA and MD, while collagen (red circles), glycosaminoglycans (blue triangles), and cell density (pink triangles) have moderate correlations with DTI-derived metrics.
We also imaged fresh excised atherosclerotic plaques prior to mechanical and histological characterisation and identified that DTI-derived tractography highlights microstructural alignments that are more, and less, vulnerable to rupture3.
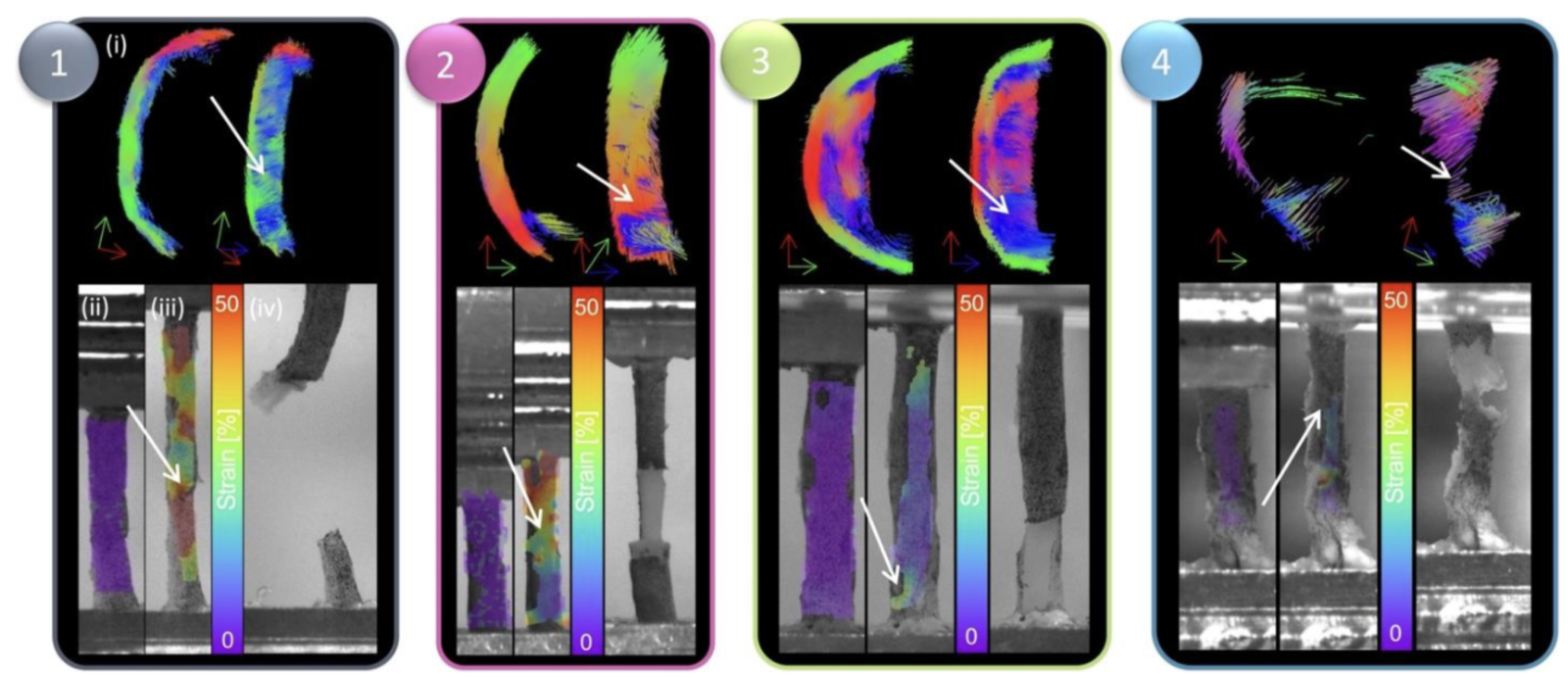
Figure 3. DIC strain contours and failure insights based on tractography groupings. For each grouping, (i) representative tractography is shown at the top, alongside strain contours on the DIC images at the (ii) reference frame (after preconditioning) and (iii) right before failure and a (iv) high-resolution image of the specimen right after failure. White arrows point to location of failure both on tractography and on strain maps.
Coming back to the initial sensitivity to cell content within arterial tissue, we also investigated tissue engineered vascular grafts. Using terminal time points and fixing the grafts on day 3, 7, and 14 of culture we imaged acellular and recellularised grafts. DTI-derived metrics, FA, MD, and tractography, can track both the recellularisation over time as well as differentiate between acellular and cellular grafts4.
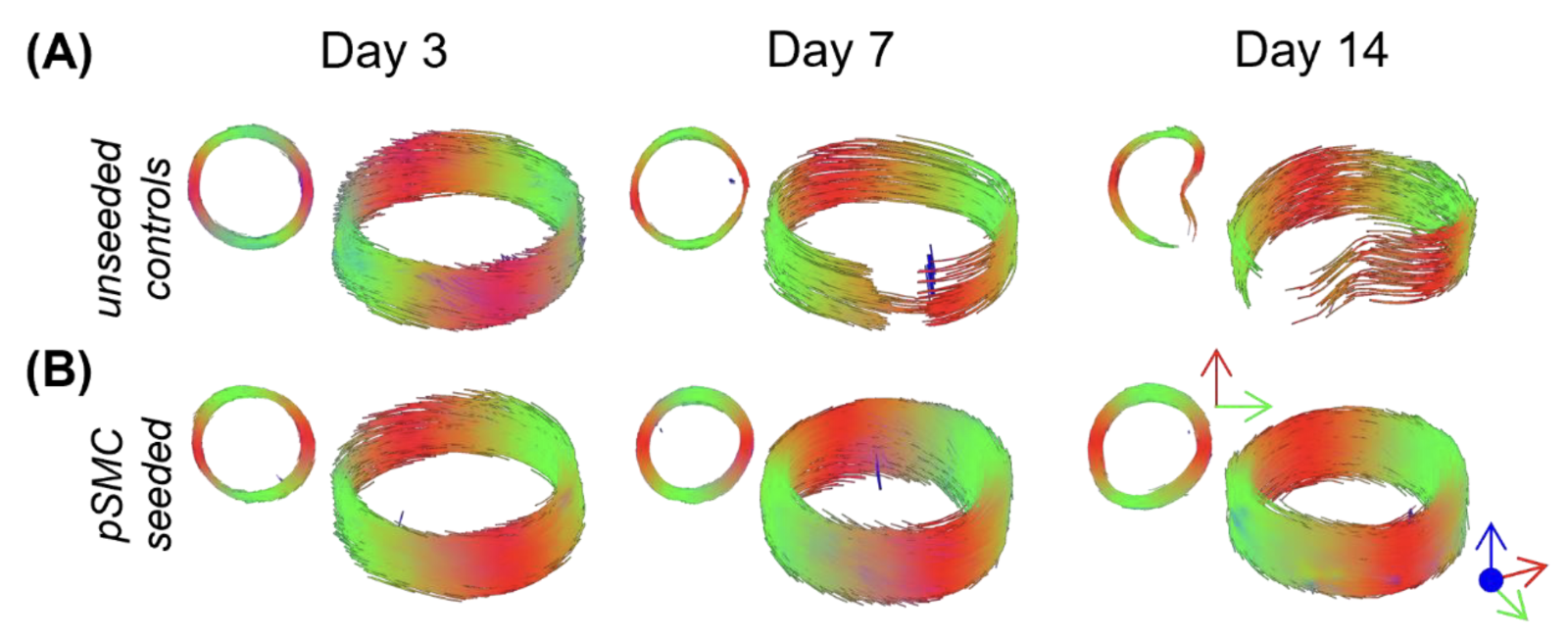
Figure 4. Tractography of tissue engineered vascular grafts. (A) Unseeded control vessels are shown in the top row, with representative (B) recellularised grafts in the second row.
Quantitative susceptibility mapping in arterial tissue:
With an aim to investigate a more clinically relevant MRI sequence which also is sensitive to the microstructure in arterial tissue, quantitative susceptibility mapping is also being explored within arterial tissue models. In porcine arterial tissue, a strong sensitivity to collagen content was linked to the susceptibility of the tissue. Human carotid arteries were also explored ex vivo5.
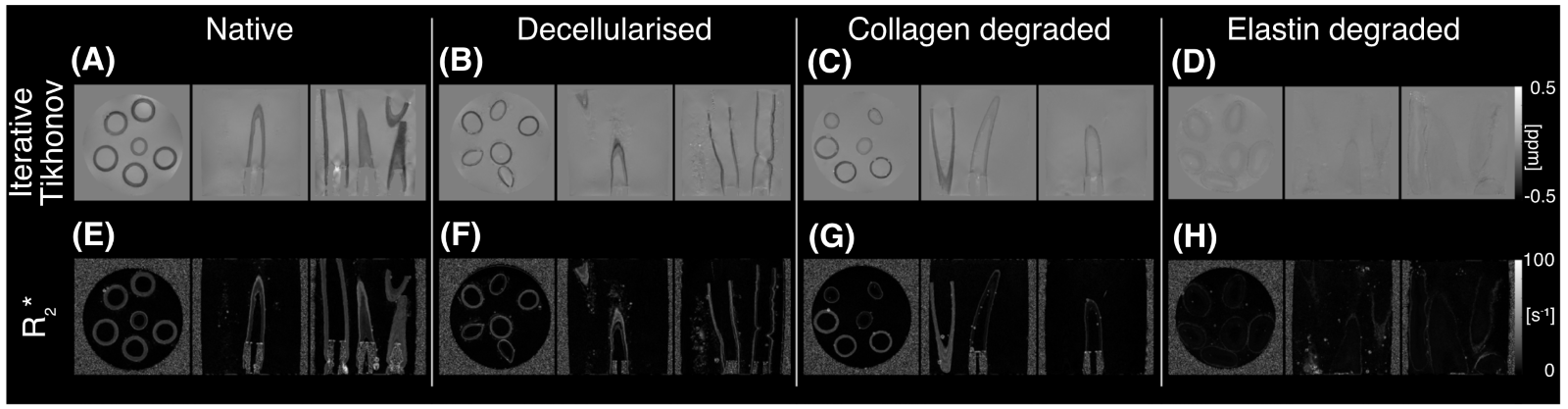
Figure 5. Susceptibility maps produced for the different porcine carotid tissue models (native, decellularised, collagen-degraded, and elastin-degraded) using the iterative Tikhonov approach (A- D) alongside maps of R∗2 (E- H).
We also imaged fresh human atherosclerotic plaques using QSM to investigate the potential of this imaging modality to provide indicators for atherosclerotic disease development. An optimised quantitative susceptibility mapping processing pipeline was developed for analysing the plaque tissue ex-vivo data. Current studies on this area are ongoing.
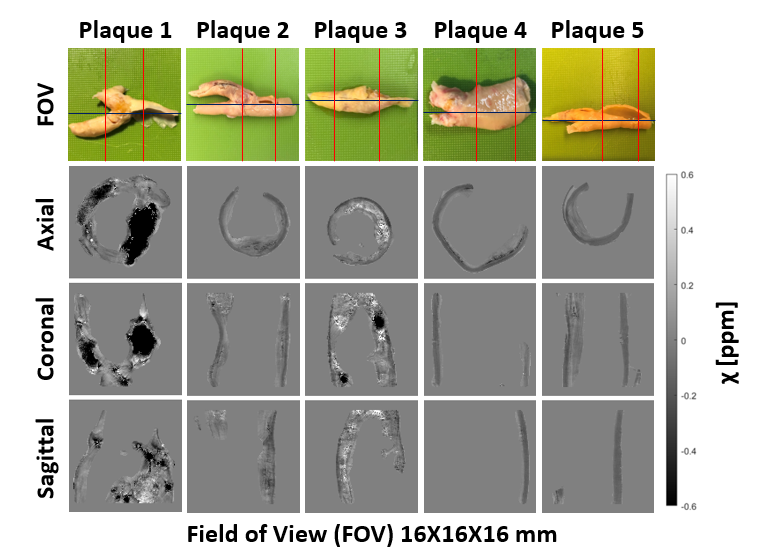 Figure 6. Quantitative susceptibility maps of five fresh excised atherosclerotic plaques. Field of view (FOV) for each plaque highlighted by red lines on the specimen pictures (first row). Axial (second row), coronal (third row), and sagittal (forth row) view of the respective quantitative susceptibility maps.
Figure 6. Quantitative susceptibility maps of five fresh excised atherosclerotic plaques. Field of view (FOV) for each plaque highlighted by red lines on the specimen pictures (first row). Axial (second row), coronal (third row), and sagittal (forth row) view of the respective quantitative susceptibility maps.
Publications1–6
1. Tornifoglio, B. et al. Diffusion tensor imaging and arterial tissue: establishing the influence of arterial tissue microstructure on fractional anisotropy, mean diffusivity and tractography. Sci. Rep. 10, 1–12 (2020).
2. Tornifoglio, B., Stone, A. J., Kerskens, C. & Lally, C. Ex Vivo Study Using Diffusion Tensor Imaging to Identify Biomarkers of Atherosclerotic Disease in Human Cadaveric Carotid Arteries. Arterioscler. Thromb. Vasc. Biol. 42, 1398–1412 (2022).
3. Tornifoglio, B., Johnston, R. D., Stone, A. J., Kerskens, C. & Lally, C. Microstructural and mechanical insight into atherosclerotic plaques: an ex vivo DTI study to better assess plaque vulnerability. Biomech. Model. Mechanobiol. (2023) doi:10.1007/s10237-022-01671-5.
4. Tornifoglio, B. et al. Exploring DTI-derived metrics to non-invasively track recellularisation in vascular tissue engineering. BioRxiv Prepr. Serv. (2022).
5. Stone, A. J. et al. Quantitative susceptibility mapping of carotid arterial tissue ex vivo: Assessing sensitivity to vessel microstructural composition. Magn. Reson. Med. 00, 1–16 (2021).
6. Shahid, S. S. et al. Exploring arterial tissue microstructural organization using non-Gaussian diffusion magnetic resonance schemes. Sci. Rep. 11, 1–13 (2021).